More than 125 years ago, Vienna medical student Ernst Freund noticed a strange phenomenon in some of his patients. Similar to diabetics, those with cancer had an “abnormal sugar content” in their blood that disappeared after surgical removal of the tumor. Some decades later, as a professor, he and others showed that compared to normal cells, cancer cells have a particularly sweet tooth in the sense that they would take up large amounts of glucose from culture medium which would stimulate their rapid growth. The most famous experiments were conducted by Otto Warburg and his colleagues in the 1920s at the Kaiser-Wilhelm Institute for Biology in Berlin. Warburg, a German biochemist and later Nobel laureate, had shown that tumor cells distinguish themselves from almost all normal cells through their preference to ferment glucose to lactate in a process known as glycolysis (1, 2).

Usually, in the presence of sufficient oxygen, normal cells prefer to completely oxidize glucose to water and CO2 through respiration, a much more efficient way for producing energy than fermentation. Only if oxygen supply is limited, like in muscle cells during a sprint, such cells would switch to fermentation of glucose for rapid energy production with the end product lactate that everybody who has once done some all-out sprints surely connects to the “burning” sensation during such an effort. After the sprint, however, an increased breathing frequency would quickly lead to re-oxygenation and a switch back to respiration in the muscle cells. But tumor cells are different. They ferment glucose to lactate independent of the oxygen supply, a phenomenon therefore known as aerobic glycolysis or the Warburg effect (3). This effect is the basic principle behind positron emission tomography with (18F)-fluorodeoxyglucose (FDG), a radioactive tracer that enters cells just like glucose but then accumulates within the cell until radioactive decay because it cannot be degraded any further. FDG PET is now routinely used as a tumor staging tool, because in general (as also already noted by Warburg) the more aggressive a cancer cell the more glucose, and hence FDG, it tends to take up.
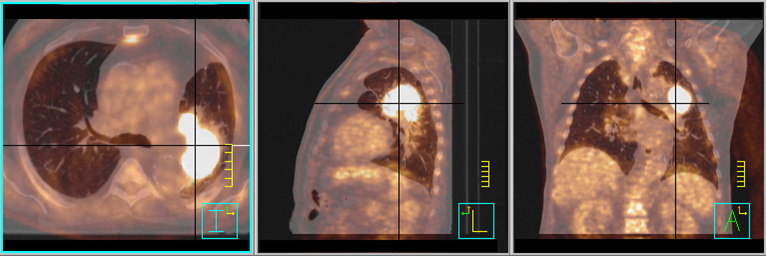
Both the Warburg effect and Ernst Freund’s observations about high glucose levels in cancer patients suggest that – simply speaking – there is a link between sugar and cancer. Furthermore, it is now well established that persons with the metabolic syndrome characterized through overweight, high blood glucose and insulin levels have a higher risk for developing, and dying from, cancer of various origins (4). The obesity epidemic together with the broad application of FDG-PET in clinical practise has led to a renewed interest in the metabolism of cancer cells and their tumor-bearing host as well as the interaction between them (5). This has been accompanied by a paradigm in which cancer can be seen more as a metabolic than a purely genetic disease (6). Because nutrition is a major modulator of whole-body as well as cellular metabolism, one could logically ask whether the type of nutrition can have an impact on cancer initiation and progression. Again, already 100 years ago scientists had addressed this question in feeding experiments with rodents. And again it was sugar, or more generally carbohydrates, that after digestion are broken down to glucose molecules, that seemed to fuel tumor growth. When rats were fed solely with milk protein and lard, their implanted tumors grew much slower than those of their littermates on standard rodent chow (7). The same, however, was observed when animals were calorie, but not necessarily carbohydrate, restricted (8). During the last couple of years, a large number of mouse studies have confirmed that both calorie restriction and ketogenic diets can delay the emergence of tumors and slow down tumor growth (9).
The ketogenic diet is characterized through a very low carbohydrate and compensatory high fat content which leads to low blood sugar and insulin levels and subsequently to the production of ketone bodies from fatty acids. Ketone bodies are energetic substrates that are readily taken up by muscles and most other tissues and can substitute for most of the glucose that is needed by the brain. This state, termed “ketosis”, is the same that occurs during prolonged starvation. In fact, the effects of starvation on hormones, blood glucose levels, mineral and water balance are mediated mainly by the absence of carbohydrates and are very similar to those of a ketogenic diet (10, 11). KDs were actually developed in the 1920s as a method of mimicking fasting while avoiding malnourishment in the treatment of epilepsy (12).Several mechanisms have been proposed to explain the limiting effects of the ketogenic diet on tumor growth. The most obvious one is the reduction of blood sugar. Indeed, if you deprive cultured cancer cells from glucose, they quickly die. However, in the living organism things are a little bit more complicated. Even with zero carb intake, blood sugar will not reduce to zero, but remain at a stable low level. Hence you cannot expect to “starve” a tumor to death, the more so as tumor cells often outperform their normal counterparts when it comes to efficient glucose uptake. In some studies, mice on a ketogenic diet had to be additionally calorie restricted in order to lower their blood sugar levels and retard tumor growth; in others, blood sugar on the ketogenic diet was not different from the control animals, yet tumos grew slower. However, given the increased risk of diabetics to fall victim to cancer as well as the shorter life expectancy of cancer patients who have high blood sugar, other indirect effects of blood glucose might play a role. Indeed, high blood glucose levels are known to trigger an inflammatory response, and inflammation is a powerful driver of both tumor initiation and development (13). Hence, chronically elevated blood glucose levels might predispose a person to cancer by inducing a chronic low-level inflammatory state. It is also well known that glucose stimulates the release of insulin from the islet cells of the pancreas. Insulin binds to specific receptors on a cell’s surface to initiate a signalling cascade that not only has metabolic consequences such as increased glucose and decreased fat oxidation, but also can affect how genes are transcribed. In insulin-sensitive cells, this hormone stimulates glucose uptake and storage as well as glycolysis. Insulin is also a potent signal for cell survival, growth and duplication. Imagine that each cell’s DNA suffers 100,000s of damaging processes each day simply through normal metabolic processes or other insults such as solar radiation. Normally, these so-called lesions get repaired before the cell undergoes division; however, sometimes repair fails and a genomic mutation results. Cells that have accumulated too many mutations in their genome normally commit suicide or go into a state of permanent dormancy. However, when things go bad, DNA damage or mutations can also lead to increased cell division and initiate tumor formation. The factors that make things go bad include inflammation, but also high concentrations of growth factors such as insulin (14). Thus, high levels of insulin in the microenvironment of a pre-cancerous cell could make the difference between survival and suicide. This effect has often been proposed to explain the increased risk of diabetics for cancer. Given the consumption frequency and high carbohydrate content of the typical Western diet, insulin levels in persons consuming such diets will be elevated over most of the day, providing a growth-stimulating environment for tumor cells. Insulin also stimulates the liver to release the so-called insulin-like growth factor (IGF)-1 which is another potent stimulator of cell growth and survival (14). IGF-1 is also formed in fat cells and frequently elevated in persons with the metabolic syndrome. In those persons, it can be normalized through weight loss and blood sugar reduction. Insulin and IGF-1 can be viewed as the foot on the gas pedal that keeps the car running fast. In addition, many tumor cells have genetic mutations in cellular signalling molecules downstream of the insulin or IGF-1 receptor that lead to permanent full throttle. In recent years, molecular biology has identified mechanisms how carbohydrate restriction counteracts these signalling molecules through an enzyme called adenosine monophosphate-activated protein kinase (AMPK). For a cell, AMPK is an energy sensor and gets activated either when internal energy levels fall or when the external environment signals to better save energy. Carbohydrate restriction is such a signal (15); intensive exercise, for example, is another. Again, the ketogenic diet and starvation are similar in that they both activate AMPK, thereby negatively affecting the pathways that program tumor cells to divide and survive (16).In summary, inhibition of insulin and IGF-1 signalling, activation of AMPK and a decrease in blood glucose levels could all be implicated to inhibit tumor growth in mice, although a direct effect of the latter seems unlikely in humans. In general, a transfer of these results to humans is complicated by the different mouse strains used in these studies, different diet compositions (in particular fat quality) and the fact that mice have a several-fold increased resting metabolic rate, so that modest calorie restriction would correspond to fasting in humans. Some of the mouse studies further found that the concentration of ketone bodies in the blood predicted the response of the tumors to the ketogenic diet. Is it possible that ketone bodies also have anti-tumor properties? Indeed, evidence from a few cell culture experiments implies just that. Dr. Eugene Fine, Professor of Clinical Nuclear Medicine at Albert Einstein College of Medicine/ Montefiore Medical Center, and his colleagues showed that ketone bodies inhibit glycolysis in several colon and breast cancer cell lines impacting their ability to efficiently generate energy (17). It seems that contrary to normal cells, many tumor cells lack the necessary enzymes to effectively burn ketone bodies so that they cannot compensate for energy loss when glycolysis is impaired. Recently, Fine and others published the results from a small clinical pilot study in which patients with advanced stage metastatic carcinomas went on a ketogenic diet for 4 weeks (18). Using FDG-PET scans they basically showed that the higher the ketone concentrations putty , the better the chances that no tumor progression occurred.
Unfortunately, only a few other pilot studies have been conducted in humans, but the results seem promising and certainly justify further research. A few case reports exist that document partial or full vanishing of detectable tumor mass in patients with a very bad prognosis (19, 20). In 2007 Ulrike Kämmerer, PhD at the University hospital of Wuerzburg, Germany, initiated a study to test the feasibility of a 12-week ketogenic diet in very advanced cancer patients in which standard treatment options were used up (21). Although some of the patients succumbed to their disease during the intervention period, some reached a state of stable disease and quality of live improved in many. Quality of life is an area where ketogenic diets could be especially beneficial, since ketone bodies are known for their muscle-conserving effects that represent an evolutionary adaption to longer-term starvation. In particular patients with intestinal tumors are predisposed to what is known as cachexia, a state of muscle and fat loss and diminished appetite. Cachexia is connected to a whole-body inflammatory state, so that avoiding additional inflammatory stimuli like high blood glucose levels seems to make sense. In addition, abundant fat intake stimulates ketone body production and supplies normal tissues like muscle with energy – energy that most tumors cannot use efficiently as they have impaired respiration (6).
Although some scientists like Thomas Seyfried, professor of biology at Boston College, advocate ketogenic diets in a calorie restricted form in order to more effectively decrease blood sugar and increase ketone levels, the associated weight loss might contradict such a diet in certain cases. On the other hand, since overweight seems to worsen the prognosis for many tumor patients, in particular those with hormone-dependent carcinomas like breast or prostate cancer, calorie restricted ketogenic diets might be superior to all-you-can-eat ketogenic diets. However, even the latter seem to promote weight loss in the obese, often to a greater extent than low-fat calorie restricted diets. In addition, low carb diets have been shown to improve several markers of inflammation, implying that carbohydrate restriction could possibly even aggravate cancer initiation and progression to a detectable disease (9).
Several clinical trials in the US are now recruiting patients for supportive nutritional therapy using either ketogenic diets or calorie restriction. In Germany, a new study called the KOLIBRI trial has just started recently and is going to compare a ketogenic diet, a moderately low carb diet and a diet following the official guidelines in breast cancer patients. Hopefully, these interventions will confirm the promising results that already exist and spread the word to the many physicians who still believe that fat is an unhealthy nutrient that hardly could help in fighting this fatal disease.
References:
1. Warburg O. Über den Stoffwechsel der Carcinomzelle. Klin. Wochenschr. 1925:534–536.
2. Warburg O, Wind F, Negelein E. Über den Stoffwechsel der Tumoren im Körper. Klin. Wochenschr. 1926:828–832.
3. Koppenol WH, Bounds PL, Dang C V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337.
4. Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43.
5. Dang C V. Links between metabolism and cancer. Genes Dev. 2012;26:877–90.
6. Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab. 2010;7:7.
7. Van Ness van Alstyne E, Beebe SP. Diet studies in transplantable tumors. I. The effect of non-carbohydrate diet upon the growth of transplantable sarcoma in rats. J Med Res. 1913:217–232.
8. Tannenbaum A. The genesis and growth of tumors. II. Effects of caloric restriction per se. Cancer Res. 1942:460–467.
9. Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab. 2011;8:75.
10. Klein S, Wolfe RR. Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol. 1992;262:E631–E636.
11. Westman EC, Mavropoulos J, Yancy WS, et al. A Review of Low-carbohydrate Ketogenic Diets. Curr Atheroscler Rep. 2003;5:476–483.
12. Wilder RM. The effect of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921;2:307.
13. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
14. Djiogue S, Kamdje AHN, Vecchio L, et al. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20:R1–R17.
15. Yamada KA. Calorie restriction and glucose regulation. Epilepsia. 2008;49:1528–1167.
16. Champ CE, Baserga R, Mishra M V, et al. Nutrient restriction and radiation therapy for cancer treatment: when less is more. Oncologist. 2013;18:97–103.
17. Fine EJ, Miller A, Quadros E V, et al. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell Int. 2009;9:14.
18. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28:1028–1035.
19. Nebeling LC LE. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Diet Assoc. 1995;95:693–697.
20. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab. 2010;7:33.
21. Schmidt M, Pfetzer N, Schwab M, et al. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab. 2011;8:54.